ethanol dielectric constant
Relative permittivity is the ratio of the permittivity of a substance to the permittivity of space or vacuum. F dielectric constant bronyl chloride 94 521 butane 30 14 butanol 1 68 178 butanone 68 185 butycic anhydride 20 12 butyl chloral 64 10 butyl chloride 68 96 butyl oleate 77 4 butyl stearate 80 31 butylacetate 66 51 butylamine 70 54 butyraldehyde 79 134 butyric acid 68 3 butyric anhydride 68 12.

Dielectric Constant Of Polycaprolactone Solvents 33 Download Table
Eds Supplement to IV6.

. If enough ethanol is added the electrical attraction between phosphate groups and any positive ions present in solution becomes strong enough to form stable ionic bonds and DNA precipitation. The excess parameters have been fitted. Relative permittivity can be expressed as.
The ratio between the actual material ability to carry an alternating. Knowing the Dielectric Constant k of a material is needed to properly. 255 rows The constant is.
Burdick Jackson solvents are arranged in order of increasing dielectric constant the ratio of the electrical capacity of a capacitor filled with the solvent to the electrical capacity of the evacuated capacitor at 20C unless otherwise indicated. Dielectric constant of the mixture 1 water. 1a the dielectric constant of Ethanol is higher at l00kHz ie.
The relative permittivities of seven binary mixtures of methanol with ethanol isomers of propanol and butanol are reported for various mole fractions at 28815 29315 29815 30315 and 30815 K. This means that adding ethanol to solution disrupts the screening of charges by water. 1984 1908 and 1829 at 10C 20C 30C 40C and 50C respectively and decreases uniformly as the frequency increased and becomes almost constant at the highest frequency of 50 MHz.
List of dielectric constants Substance Substanz Dielectric Constant Cyanogen Cyan 25 Decalin Decalin 21 Degalan Degalan 31 Desmodur Desmodur 100 Diacetone alcohol Diacetonalkohol 182 Diamylether Diamylether 30 Diatomaceous earth Kieselgur 14 Dibenzofuran 100C Dibenzofuran 100C 30 Dibenzyl 60C Dibenzyl 60C 25. The dielectric constant - also called the relative permittivity indicates how easily a material can become polarized by imposition of an electric field on an insulator. Dielectric Constant k is a number relating the ability of a material to carry alternating current to the ability of vacuum to carry alternating current.
εr ε ε0 1 where. Bromine 31 Ethanol ethyl alkohol 162 Butanoic acid 3 Ethayl acetate 6 Ethyl benzene 24 Cacao beans 18 Ethyl benzoate 6 Calcium fluoride 25 Ethyl mercaptan 69 Camphene 23 Ethylamine 69 Caproic acid 26 Ethylene chlorhydrin 25 C D E B. Diethanolamine C4H11NO2 CID 8113 - structure chemical names physical and chemical properties classification patents literature biological activities safety.
Ethanol is much less polar than water with a dielectric constant of 243 at 25 C. The dielectric constants relative permittivities of water methanol ethanol butanol and acetone were measured at 913 kPa and 28315 and 29315 K and are reported here. Dielectric constants of common materials materials deg.
The dielectric constants were determined by using a new setup based on a. Dielectric constants of common liquids H2O water 785 HCOOH formic acid 585 HCON CH3 2 NN-dimethylformamide 367 CH3OH methanol 327 C2H5OH ethanol 245 CH3COCH3 acetone 207 n-C6H13OH n-hexanol 133 CH3COOH acetic acid or acetic acid 615 C6H6 benzene 228 CCl4 carbon. We have computed the static dielectric constant and the trans-verse and longitudinal components of the dielectric permit-tivity at different wave-vector numbers paying special atten-tion to the low wave-vector limit which provides an alternative route to calculate the dielectric permittivity.
Obtained for liquid ethanol by means of MD simulations. Surface tension and dielectric constant decrease in the order water ethanol 52 and ethanol 80 3134 35 while molar volume increases in this. Given the negligible difference in the value of the dielectric constant of water at 20 degrees C and that of ethanol solutions at low temperatures the often advanced explanation for the precipitation of plasma proteins by the cold ethanol process as being due to a reduction of the dielectric constant and the resulting increase in.
The capacitance created by the presence of the material is directly related to the Dielectric Constant of the material. The excess dielectric permittivity the temperature coefficients lnεrT and their excess values were calculated. Empirical Formula Hill Notation.
Landolt-Börnstein - Group IV Physical Chemistry vol 17.

Dielectric Constants At Room Pressure For Water 5 6 12 At 298 K And For Download Table

Hildebrand Hansen Solubility Parameters And Dielectric Constants For Download Table

The Dielectric Constant Of Water And Some Organic Solvents Download Table

Polarity Indexes Of Ethanol Dmso And Chloroform According To Their Download Scientific Diagram

The Changes In The Dielectric Constant For 40 10 And 0 5 Ethanol Download Scientific Diagram

Extracted Dielectric Constants Of Chemicals In 13 18 Ghz Download Table

Dielectric Constants E Of Solvents And Li 6 A P 2 W 18 O 62 And K Download Table

Dissipation Factor And Dielectric Constants For Some Solvents Commonly Download Table

List Of Structure Dielectric Constant And Boiling Point Of Dispersion Download Table

Boiling Point Dielectric Constant E And Dielectric Loss Tangent Tan Download Table

The Changes In The Dielectric Constant For 40 10 And 0 5 Ethanol Download Scientific Diagram

Dielectric Constant Of Five Ethanol Water Mixtures Download Scientific Diagram

List Of Structure Dielectric Constant And Boiling Point Of Dispersion Download Table

Comparison Of The Obtained Dielectric Constant Data With Values Download Table

Extracted Dielectric Constants Of Chemicals In 13 18 Ghz Download Table
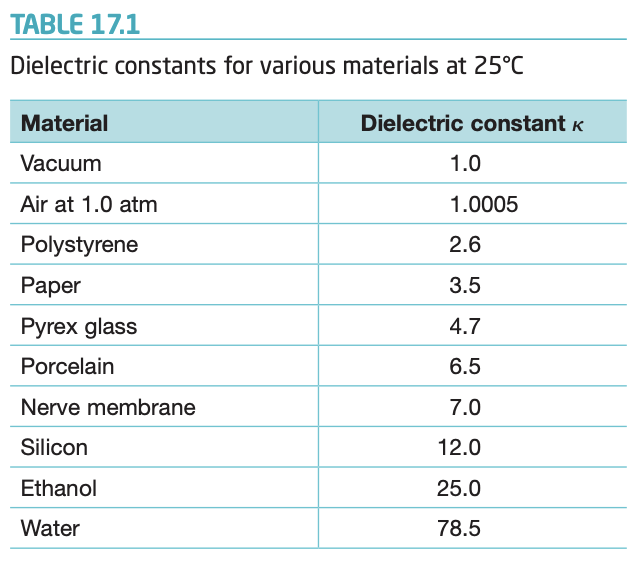
Solved Table 17 1 Dielectric Constants For Various Materials Chegg Com

Pdf Dielectric Constants Of Water Methanol Ethanol Butanol And Acetone Measurement And Computational Study

Experimental And Theoretical Dielectric Constant For Toluene Ethyl Download Scientific Diagram

Estimated Static Dielectric Constant E0 And Relaxation Time T For Download Table
Comments
Post a Comment